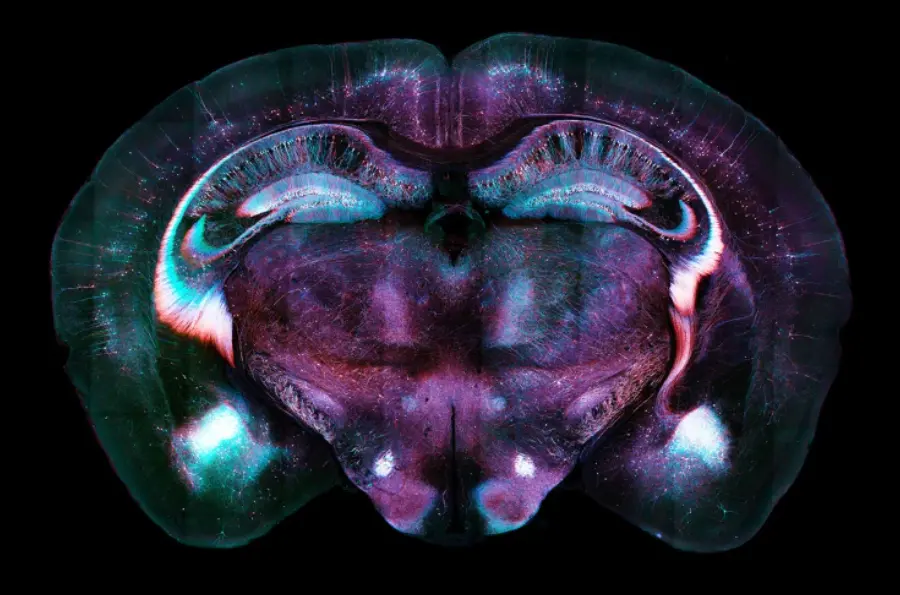
In a breakthrough, scientists at the Massachusetts Institute of Technology (MIT) have found a particular brain circuit in mice that enables the process of “fear extinction”—a neurological phenomenon in which fear memories are increasingly overcome by safety signals. This discovery has the potential to revolutionize the treatment of post-traumatic stress disorder (PTSD), a disorder affecting millions worldwide.
What Is Fear Extinction?
Fear extinction is the learning process by which a person comes to understand that a stimulus previously linked with threat is no longer dangerous. For instance, a war veteran may initially be filled with terror at the sound of fireworks, but repeated exposure to such sounds in secure settings can eventually eliminate this response. In many people, this occurs automatically. But in people with PTSD, the fear response continues—often with debilitating effects.
Until now, the neurobiological basis of this process was poorly understood. While scientists have long known that areas like the amygdala and prefrontal cortex are involved in fear processing, the specific mechanisms triggering the brain to suppress conditioned fear remained elusive.
The Dopamine “Safety Signal”
The MIT researchers, under Dr. Kay Tye, were interested in dopamine’s contribution to fear processing. Dopamine is classically associated with reward and motivation, but researchers found that in specific neural circuits, dopamine functions as a kind of “safety signal” during fear extinction.
The most important pathway is one in which neurons extend from the ventral tegmental area (VTA) to the nucleus accumbens. Mice released dopamine in this pathway when they were exposed to something they had learned to associate with fear—a tone followed by a weak shock—but no longer received the shock. The release of the dopamine communicated that the danger had subsided, allowing the brain to extinguish the fear response.
To verify their results, researchers employed optogenetics—a technology that employs light to manipulate neurons—to activate and silence the dopamine signals. Mice that received increased dopamine signaling experienced faster fear extinction, but those with silenced dopamine release found it hard to learn that the environment was secure.
Bridging the Gap to Human PTSD Treatment
The therapeutic implications for treating PTSD are profound. Classical exposure therapy, the most potent PTSD treatment, depends on the brain’s intrinsic ability to extinguish fear. When that system malfunctions, however, therapy becomes less effective. By manipulating this dopamine-mediated circuit, clinicians might be able to enhance the efficacy of therapy for recalcitrant cases.
Dr. Tye adds, “The concept is not to suppress memories of traumatic events, but to assist the brain in updating its knowledge of what is safe. If we can reproduce this process in humans, we can provide a lifeline for those caught in the cycle of fear.”
Other Mechanisms and Interactions
This finding is consistent with and extends earlier research that indicates that other neurotransmitter systems are also involved in fear extinction. For example, acetylcholine and glutamate have been shown to be involved in the modulation of attention and memory during fear-learning processes. Additionally, astrocytes, non-neuronal brain cells previously believed to have only a supportive role, are increasingly found to be active participants in modulating neural circuits during fear extinction.
Indeed, a recent study funded by NIH demonstrated that cholinergic transmission to hippocampal astrocytes regulates natural fear extinction, and pharmacological interventions in glial cells might also augment dopamine-based therapies.
This implies that fear extinction is a multi-component, multi-faceted process that engages both chemical and structural brain elements—a reassuring finding for scientists looking for multiple points of therapeutic entry.
The Road Ahead
Although the results in mice are promising, much has to be done before they can be applied to human treatments. Scientists are now investigating non-invasive means of boosting dopamine activity in corresponding brain areas in humans, possibly with transcranial magnetic stimulation (TMS), deep brain stimulation, or targeted drugs.
In addition, the group is working with clinicians to investigate whether PTSD patients show differences in dopamine signaling during exposure therapy. If that is the case, treatments may one day be tailored according to an individual’s neurochemical signature—a first toward personalized psychiatric medicine.
The research also presents ethical concerns regarding manipulation of memory and control of emotions. As much as extinction of fear is a natural and mandatory component of psychological recovery, the issue of emotional memory circuits manipulation must be approached with sensitivity.
Conclusion
The discovery of a dopamine-mediated “fear extinction” signal in mice represents a breakthrough in neuroscience with deep implications for the treatment of PTSD. By opening the door to the neural processes that inform the brain that it is safe, scientists are taking us closer to more effective, tailored therapies for those who suffer from the debilitating legacy of trauma.
As science continues to untangle the complex architecture of fear, emotion, and memory, the future of mental health care appears ever more promising—and grounded in the brain’s own elegant complexity.